The lewis concept
The
Lewis Concept (G.N. Lewis 1923)
Lewis
acid - species which can
accept electrons (Electron acceptor)
Lewis
base - species which can
donate electrons (Electron donor).
A lewis acid - base reaction
results in the formation of a coordinate covalent bond between the two species.
Generally, lewis acid is identified
by the presence of vacant orbitals which can
accommodate electrons whereas a lewis
base will have lone pair of electrons available for shairing.
Thus, lewis definition extends
beyond reactions which involve protons. Its most important application is in
describing complex ion formation e.g.
The strength of the
base is in a given solvent depends on the nature of the acid used as a
reference.
Note:Brfnsted-Lowry
base (accepts a proton by donating e’s)
is also a Lewis base BUT a
Brfnsted
-Lowry acid is not necessarily a Lewis acid.For reactions which
occur in aqueous solution, Brfnsted
-Lowry definitions are more applicableIn systems ;which do
not involve protons, Lewis definitions is more valuable
Classes
of solvents
w.r.t Brfnsted-Lowry
acid-base properties, solvents are either aprotic
or amphiprotic. Aprotic solvents are neither
acidic nor basic , e.g. CCl4, C6H6
Amphiprotic solvents - can act
as both proton acceptors and proton donors. They range from predominantly
acidic to predominantly basic. Water and ethanol are neither strongly acidic or
strongly basic.
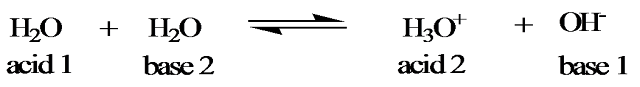
All Amphiprotic solvents undergo Autoprotolysis or self ionization
reaction:(spontaneous reaction of molecules to give pair of ions)
Where SH2+ is
solvated proton;
Self ionization (Autoprotolysis) of water
Water exhibits both
acidic and basic properties:
The reverse reaction
proceeds to a greater extent than the forward reaction and therefore the
equilibrium lies to left.
K = [H3O+][OH-] = Kw
Kw –
ion product of water
At 25oC,
Kw =
10-14.
In Pure water, [H3O+] = [OH-] = 10-7
Note: self ionization of
water contributes to the hydrogen (hydronium ion) and hydroxide ion concentration in solution but
the effect depends on the concentration and the nature (strong/ weak acid/ base) of
the solute. In General, if [solute] > 10-6
M, the contribution of either OH- or H3O+
is negligible
pH
Scale;Potential of
hydrogen ion” is the – ve potential of the hydronium ion
pH = -log [H3O+]
Examples
Calculate pH of 0.005
M HNO3
acid,
pH = -log [H3O+] = -log 0.005 = 2.3
Calculate [H3O+] for HCl solution at pH 3.5:
3.5 = -log [H3O+]; [H3O+] = antilog -3.5 =
3.16 X 10-4 M
Similarly, pOH = -log [OH-]
Thus
Kw =
[H3O+][OH-] = 10-14 Þ [H3O+] = [OH-] = 10-7
-Kw =
-([H3O+][OH-])
-log Kw =
-log [H3O+] + -log [OH-]
Since -log [H3O+] = pH and -log [OH-] = pOH
pKw = pH + pOH =14
hence for a neutral
solution, pH = pOH = 7
Note:
Self ionization of
water contributes to the amount of H3O+
and OH-;
The overall effect of self ionization of water depends on whether the acid or base is a strong or weak
electrolyte
Consider 0.01 M HNO3,
both the HNO3
and H2O
contribute H3O+
But nitric acid
(strong acid) dissociates completely to produce 0.01 M H3O+.
By Le Chatelier’s principle, the
amount of H3O+
(from autoprotolysis of water) will be
diminished at equilibrium and will be expected to be less than 10-7
M. Thus the contribution of H3O+ from water will be negligible.
Numerically,
For 0.01 M HNO3 ,
[H3O+] = 0.01 M; At
equilibrium,
Kw = [H3O+][OH-] = 10-14
Thus [OH-] <<< 10-7 ([OH-] in pure water.
Since the only source
of OH-
is self ionization water, [H3O+] contributed by water = 10-12
M. Thus the contribution of [H3O+] contributed by self ionization of water is negligible.
Similarly, for strong
base e.g. 0.01 M NaOH, the contribution of
self ionization of water to [OH-] is negligible as it
is less than 10-7 M
In 0.01 M NaOH, [OH-] = 0.01 M
Thus [OH-] from self ionization
of water = 10-12 M
Generally, if the dissolved
acid or base contributes a [H3O+] or [OH-] ³ 10-6 M, the contribution of water to the total concentration
of either H3O+
or OH-
is negligible.
Examples
1.The pH of rainwater
is found to be 4.35 while that of ammonia solution is found to be 11.28.
calculate the concentration of the hydronium and hydroxide ions in (a) rainwater and (b) ammonia
solution
2. What volume of water
is required to raise the pH of 1.00 L of HCl
solution from 2.5 to 3.10
3. The solubility of
Ca(OH)2
is 0.165 g/ 100 ml of solution at 20 °C. What is the pH of
the saturated solution of calcium hydroxide at 20 °C.
4. Calculate the pH of
a solution that is 3.00% KOH by mass and has a density of 1.0242 g/ml
Weak acids and Weak bases
Partially ionized in
solution
For weak acid, HA, which dissociates according to
The
equilibrium constant, known as acid ionization constant, Ka, is
given by;
For weak
base, BH
For
acetic acid, CH3COOH
pKa = -log Ka = -log
1.8 X 10-5 = 4.74
The larger the Ka ,
the farther the equilibrium lies to the right and greater the concentration of
the anion produced.
Examples
1. A 0.250 M aqueous
solution of butyric acid showed a pH of 2.72. Determine the acid dissociation
constant of the organic acid.
solution
Init. Conc 0.250 M - - -
D -x M x M x M
Equi.Conc (0.250-x) M x M x M
pH = -log [H3O+]
[H3O+] = antilog 2.72 = 1.9
X 10-3 =
x
Qtn Cocain (C17H21O4N)
is an alkaloid, with a characteristic bitter taste, has a solubility of 0.17g/
100 ml. If the pH of the saturated solution is 10.08, calculate the Kb
value for cocaine.
References
·
Christian Laurence and Jean-François Gal "Lewis Basicity and
Affinity Scales : Data and Measurement" Wiley, 2009.
·
Lewis, G.N., Valence and the Structure of Atoms and Molecules (1923) p.
142.
·
Miessler, L. M., Tar, D. A., (1991) p166 - Table of discoveries attributes
the date of publication/release for the Lewis theory as 1923.
March, J. “Advanced Organic
Chemistry” 4th Ed. J. Wiley and Sons, 1992: New York
·
Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements
(2nd Edn.), Oxford:Butterworth-Heinemann.
·
Jensen, W.B. (1980). The Lewis acid-base concepts : an overview.
New York:.
· Yamamoto, Hisashi
(1999). Lewis acid reagents : a practical approach. New York: Oxford
University Press.

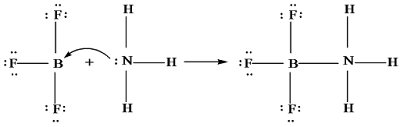













No comments: