IONIC EQUILIBRIA
IONIC
EQUILIBRIA
Ionic
equilibria in Aqueou;s Solutions Equilibria between ionic species in aqueous solutions finds
applications in industrial, analytical and physiological chemistry;Employs principles of chemical equilibria
Acid-Base
Equilibria
Arrhenius
Theory of acids and bases
Acid - species which
yields hydrogen ions in solution e.g. HCl

Base - species which
yields hydroxyl ions in solution e.g. NaOH
Thus, neutralization
reaction can be represented by the net equation
Acid base water
Equation captures the
essential
idea of the Arrhenius
theory: neutralization
reaction involves the
combination of Hydrogen and hydroxide ions
Merits
accounts for observed
electrical conductivity and colligative properties of
aqueous solutions,explains the
catalytic activity of acids in certain reactions
Limitations
Does not take into
account the role of a solvent in the ionization process and hence it faces
difficulty in the treatment of weak bases e.g. ammonia: ammonia is not treated as a base as it
contains no OH- ion. secondly,Can not account for behaviour in which a species
acts as both acid and base. thirdly,Overwhelming evidence
suggests that H+ does not exist in aqueous solution. More
realistically, H+
exists as H3O+ on account of its smaller size and
hence well accommodated by the solvent to form a hydrated species (H3O+)
Brfnsted - Lowry concept (1923)
Acid - species having
tendency to lose or donate a proton
Base
- species with a
tendency to accept or add a proton
Note:Water is capable of
acting as both an acid
and base (amphiprotic); Reaction is
reversible
Strength
of acids and bases
By Brfnsted -Lowry definition
A
strong acid - has a strong
tendency to transfer a proton to another species;A
strong base - has a high
affinity for protons;Acid strength can be
measured quantitatively by the degree to which reactants are converted to
products
The extent to which
this reaction proceeds to products is governed by tendency
of acid 1 to
lose a proton AND tendency
of base 2 to accept a proton.
Quantitative measure
of the acid strength is the acid dissociation constant, K of the reaction
THUS
Common ion effect in acid-base equilibria
Consequences of presence of an ion
which is also produced by the ionization of weak acid or weak base
Solution
of weak acids and strong acids
Suppose a solution
contains 0.100 M ethanoic acid and 0.100 M HCl. If Ka =
1.8 X 10-5 M
init. Conc
0.100 M - - -
D -x M x M x M
Equi. Conc (0.100-x) M x M x M
Assume x <<<
0.100, (0.100 - x) @ 0.100
Thus, x2 =
0.100 X 1.8 X 10-5
x = [H3O+] =
=1.3 x 10-3 M
[H3O+]
and [CH3COO-]
= 1.3 X 10-3 M
in absence of HCl.
BUT, in presence
of 0.100 M HCl,
init. Conc:
Weak acid 0.100 M - -
Strong acid -
0.100 M -
D -x M x M
x M
Equi. Conc (0.100 -x) M 0.100+x M x M

addition of HCl greatly reduced the
amount of acetate ion (from 1.3 X 10-3 to 1.8 X 10-5
M). Similarly, by the Le-Chateliers principle:
increasing the concentration of one of the products (common ion), shift the
equilibrium condition in the reverse (backward) direction.
Thus, the common ion
effect leads to the suppression of ionization of a weak acid.
Similarly, the common
ion OH- from
strong base suppresses the ionization of a weak base:
Addition of OH-
(a common ion) shifts the equilibrium to the left.
How
many drops of 12 M HCl would you add to 1.00 L of 0.100 M acetic acid to make
acetate concentration to 1.0 X 10-4 M
? assume 1 drop = 0.05 mls and that the volume of solution remains 1 L after dilution
.Calculate [H3O
Solution of weak acids and their salts
.Calculate [H3O
Solution of weak acids and their salts
Addition of salts of weak acids to solution of weak acids also tends to suppress ionization of weak acid due to common ion effect. The same applies to addition of salt of weak base to a weak base.+]and [CH3COO-]
in a solution containing 0.100 M CH3COOH and CH3COONa.
init. Conc:
Weak acid 0.100 M - -
Salt -
- 0.100 M
D -x M x M
x M
Equi. Conc (0.100-x) M x M (0.100+x) M
init. Conc:
Weak acid 0.100 M - -
Salt -
- 0.100
D -x M x M
x M
Equi. Conc (0.100-x) M x M (0.100+x) M
What
mass of Sodium acetate should be added to 1.00 L of 0.100 M acetic acid to
produce a solution of with pH = 5.00. Assume the volume remains constant
Note:A relationship exists
between the strength of an acid and its conjugate base.Strong acid often
forms a weak base and vice versa
e.g. HCl is a strong acid
because it has a strong tendency to lose a proton. But, its conjugate base, Cl-
has a small tendency to acquire a proton and therefore a weak base. Thus
forward reaction is strongly favoured, making forward
reaction virtually complete;In any solvent, the
strength of a base is determined by its ability to attract and hold a proton in
competition with solvent and other basic molecules.
Merits
Extends definition to
include terms other than H+ and OH-;Brfnsted - Lowry concept is more useful in quantitative treatment of acid-base reactions
References
References
· Christian Laurence and Jean-François Gal "Lewis Basicity and Affinity Scales : Data and Measurement" Wiley, 2009.
· Lewis, G.N., Valence and the Structure of Atoms and Molecules (1923) p. 142.
· Miessler, L. M., Tar, D. A., (1991) p166 - Table of discoveries attributes the date of publication/release for the Lewis theory as 1923.
March, J. “Advanced Organic Chemistry” 4th Ed. J. Wiley and Sons, 1992: New York
· Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann.
· Jensen, W.B. (1980). The Lewis acid-base concepts : an overview. New York:.
· Yamamoto, Hisashi (1999). Lewis acid reagents : a practical approach. New York: Oxford University Press.




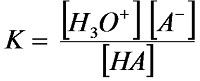




No comments: