ALKENES
ALKENES
OccurenceAlkenes (olefins) are hydrocarbons that contain a carbon-carbon double bond. Alkenes occur abundantly in nature, and many have important biological roles. For example, ethylene is a plant hormone that induces ripening in fruit, -pinene is the major component of turpentine, and -carotene is a valuable dietary source of vitamin A and is thought to offer some protection against certain types of cancer.


Ethylene is synthesized industrially by thermal cracking of natural gas (C1-C4) alkanes and straight run gasoline. Thermal cracking takes place in the absence of catalyst at temperatures up to 900OC. The high temperature reaction conditions cause spontaneous homolysis of C-C and C-H bonds, with resultant formation of smaller fragments.
Cis-Trans Isomerism
The bonding in alkenes has interesting consequences that are illustrated by the four-carbon alkenes, the butenes. Butenes exist in isomeric forms. First, in the butanes with unbranched carbon chains, the double bond may be located either at the end or in the middle of the carbon chain. Isomeric alkenes, such as these, that differ in the position of their double bonds are further examples of constitutional isomers.
The structure of 2-butene illustrates another important type of isomerism. In one form the methyl groups are on the same side of the double and is called cis-2-butene or (Z)-2-butene (b.p. 3.7OC). In another form the methyl groups are on opposite sides of the double bond and is called trans-2-butene or (E)-2-butene (b.p. 0.88OC).
These isomers have identical atomic connectivities. The two compounds, however, differ in the way their constituent atoms are arranged in space. Compounds with identical connectivities that differ in the way their constituents atoms are arranged in space are called stereisomers.

Nomenclature of Alkenes
Alkenes are named using a series of rules similar to those for alkanes, with the suffix –ene to identify the family.
1. Name the parent hydrocarbon. Find the longest carbon chain containing the double bond and name the compound accordingly.
2. Number the carbon atoms in the chain beginning at the end nearer the double bond. If the double bond is equidistant from the two ends begin at the end nearer the first branching point.

3. Write the full name, numbering the substituents according to their position in the chain listing them alphabetically. Indicate the position of the double bond by giving the number of the first alkene carbon. If more than one double bond is present, indicate the position of each and use one of the suffixes -diene, -triene and so on
.


In cyclic alkenes with a substituents, number the double bond so as to give the lowest number for the substituents at the first point of difference.
3-methylcyclohexene
|
1-Methycyclohexene
|
Substituent groups may also contain double bonds. Some widely occurring groups of this type have special names that must be learned.
The system used her is an E,Z system which is part of general system for nomenclature of stereoisomers called the Cahn-Ingold-Prelog system. The system involves assignment of relative priorities to thee two groups on each carbon of the double bond. If the groups of higher priorities are on the same side of the double, the compound is said to have the Z configuration and if they are on opposite sides of the double bond, the compound is said to have the E configuration.
To assign relative priorities proceed through the following steps:
Rule 1 Examine the atoms directly attached to a given carbon of the double bond then Assign higher priority the group containing the atom of higher atomic number (or higher atomic mass in case of isotopes.
Rule 2 If the atoms directly attached to the double bond are the same, look at the second, third or fourth atoms away from the double-bond carbons until the first difference is found. The higher priority is then assigned to the atom of higher atomic number (or atomic mass).
Rule 3 Multiple bonded atoms are equivalent to the same number of single-bonded atoms. For example, an aldehyde (-C=O), which has a carbon doubly bonded to one oxygen, is equivalent to a substituent with a carbon atom singly bonded to two oxygen atoms.
Unsaturation number
An alkene with one double bond has two fewer hydrogens than an alkane with the same carbon skeleton. Likewise, a compound containing a ring also has two fewer hydrogens in its molecular formula than the corresponding non-cyclic compound. This shows that the molecular formula of an organic compound contains information about the number of rings and double (or triple) bonds in the compound.
The presence of rings or double bonds within a molecule is indicated by a quantity called the unsaturation number, or degree of unsaturation, U. The degree of unsaturation or unsaturation number of a molecule is equal to the total number of its rings and multiple bonds. This can be calculated from the following formula;
Whereby, C is the number of carbons, N is the number of nitrogens, X is the number of halogens (Cl, Br, I, F) and H is the number of hydrogens. Oxygen is not included because its presence does not affect the number of hydrogens in a saturated compound. A halogen is monovalent and its presence replaces hydrogen. The presence of a nitrogen causes an addition of one hydrogen since it is trivalent.
Reactions of alkenes
The most common reactions of alkenes are addition reactions. The reactions of alkenes arise from the reactivity of carbon-carbon d0=+uble bond. By studying the characteristic reactions of the double bond, we can predict the reactions of alkenes we have never seen before.
Because single bonds (sigma) bonds are more stable than pi bonds, the double bond reacts and transform the pi bond into a sigma bond.
The addition reaction can be represented generally as follows:
In an addition reaction, the carbon-carbon pi bonds of the alkene and X-Y bond of the reagent are broken, and new C-X and C-Y bonds are formed.
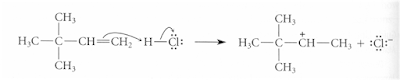
A strong electrophile (electron loving group) has the affinity to for the loosely held pi electrons and can pull them away to form a new bond leaving one of the carbon atoms with only three bonds and a positive charge: a carbocation.
§This type of reaction requires a strong electrophile to attract the electrons of the pi bond and generate a carbocation in the rate-determining step. Most alkene reactions fall into this large class of electrophilic additions to alkenes.
1. Hydrohalogenation
Markovnikov Addition of hydrogen halide
The product formed in this reaction is an alkyl halide. However when the addition is done on an unsymmetrical alkene there are two possible products. For example, the addition of HBr to 2-methyl-2-butene could lead either two products, yet only one is observed.
The first step is protonation of the double bond. If the proton add to the secondary carbon, the product will be different from the one formed if the proton adds to the tertiary carbon.

The tertiary carbocation is more stable than the secondary one, so the first reaction is favored. The second half of the mechanism shows the final product of the reaction of 2-methyl-2-butene with HBr:
Note that the proton (hydrogen) adds to the end of the double bond that is less highly substituted to give a more highly substituted carbocation (the more stable carbocation).
This rule was first stated by Vladmir Markovnikov in 1869 and is called Markonvikov’s rule. It originally stated that: The addition of a proton acid to the double bond of an alkene results in a product with the acid proton bonded to the carbon atom that already holds the greater number of hydrogen atoms. Reactions that follow this rule are said to follow Markovnikov orientation. It has now been extended to include a variety of other additions, based on the addition of the lelectrophile in such away as to produce the most stable carbocation. Markonvikov’s rule (extended): In an eletrophilic addition to an alkene the electrophile adds in such a way as to generate the most stable intermediate (carbocation).
Anti-Markovnikov Addition of hydrogen halide
Anti-markovnikov products result from addition of HBr (but not HCl or HI) in the presence of peroxides. Peroxides give rise to free radicals that act as catalysts to accelerate the addition, causing it to occur by a different mechanism.
Carbocation Rearangement
In some cases the addition of a hydrogen halide to an alkene gives an unusual product.
The minor product is the result of normal regioselective addition of HCl.
Examination of the carbon skeleton of the major product shows that a rearrangement has occurred. In a rearrangement, a group from the starting material has moved to a different position in the product.
Minor product
After the formation of the carbocation a methyl group moves (methyl shift) with its pair of bonding electrons from the carbon adjacent to the electron-deficient carbon. The carbon from which this group departs becomes electron deficient and positively charged. That is to say the rearangement converts one carbocation into another.
This is a Lewis-acid-base reaction in which the electron deficient carbon is the Lewis acid and the migrating group is a Lewis base.
It can be seen that in the carbocation rearrangement a more stable tertiary carbocation is formed from a less stable secondary carbocation. Rearangement is therefore favored by the increased stability of the rearranged ion.
A major product can also be formed by a rearangment of a carbocation to form a more stable carbocation by an immigration of a hydrogen with its bonding electrons. This is called a hydride shift.
2. Hydration of alkenes
An alkene may react with water in the presence of a strongly acidic catalyst to form an alcohol. Formally this reaction is hydration (the addition of water), with a hydrogen atom adding to one carbon and a hydroxyl group adding to the other. Hydration of an alkene is the reverse of the dehydration of alcohols.
The proton adds to the les highly substituted end of the double bond, so the positive charge appears at the more highly substituted end.
3. Halogenation of alkenes
Halogens add to alkenes to form vicinal dihalides
The reaction proceeds by formation of a halonium ion.

Examples
Halohydrins
In the addition of bromine, the only nucleopile (Lewis base) available to attack the bromonium ion is the bromide ion. When other nucleophiles are present they too can attack the bromonium to form products other than dibromides. This is common when the solvent itself is a Lewis base.
E.g. When an alkene is treated with bromine water, a water molecule attacks the bromonium ion.

Loss of proton from the oxygen gives the prolduct
The products is a bromohydrin, a member of a general class of compounds called halohydrins. These are compounds containing both a halogen and an OH group.
When the double bond is positioned asymmetrically, attack of a water molecule can give two possible products. However, the reaction is highly regioselective. When water attacks the bromonium ion, the OH group binds to the more branched carbon.
The reason for this regioselectivity is found in the structure of the bromonium ion intermediate, shown as a hybrid of three resonances structures.
When one carbon has two alkyl substituents, the centre structure, which is a tertiary carbocation, is more important than the structure on the right, which is a primary carbocation. This means that in the bromonium ion, the bond from the bromine to the carbon bearing the two alkyl groups is very weak. When water attacks the bromonium ion, it is this bond that opens, and the OH group thus binds to the more branched carbon.
5. Oxymercuration
In a reaction called oxymercuration, alkenes react with mercuric acetate in aqueous solution to give addition products in which HgOAc (acetoxy mercury) group and an OH group derived from water have added to the double bond.
THF is an important solvent because it dissolves both water and may water insoluble organic compounds. Thus, its role in oxymerucuration is to dissolve both the alkene and the aqueous mercuric acetate solution. Water is required as both a reactant and as a solvent for the mercuric acetate.

Oxymercuraation mechanism resembles halohydrin formation.
As in bromonium addition the notation can be dissected into two steps to see it relationship to other addition reactions. It can be like the attack of the alkene electrons on a Lewis acid to give a carbocaction (step 1), which reacts with the unshared electro pair on the nearby atom (Hg) to give the cyclic ion (step 2).
The mercurinium ion is then attacked by the solvent water.
Attack of the solvent occurs at the more branched carbon, just as in solvent attack on a bromonium ion.
The addition is completed by transfer of a proton to the acetate ion formed.
Conversion to alcohols
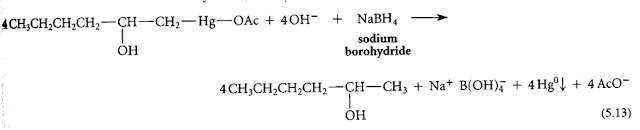
Oxymercuration is useful because its products are easily converted into alcohols by treatment with the reducing agent sodium borohydride. (NaBH4). In the presence of aqueous sodium hydroxide (NAOH).
The oxymercuration adducts are usually not isolated but are treated directly with a basic solution of sodium borohydride in the same vessel (one pot reaction). When these reactions are used sequentially, are referred to as oxymercuration-reduction. The overall result of oxymercuration-reduction is the net addition of the elements of water to the alkene double bond in a highly regioselective manner. The OH group is added to the more branched double bond.
6. Hydroboration
Borane (BH3) add regioselectively to alkenes so that the boron becomes bonded to the less branched carbon of the double bond and hydrogen becomes bonded the more branched carbon.
Because borane has three B-H bonds, one borane molecule can add to three alkene molecules.

The addition of BH3 is called hydroboration. Common solvents are ethers, diethyl ether, THF and diethyleneglycoldimethylether (diglyme).
Each of the three additions in hydroboration reactions believed to occur as a single step.
A reaction that occurs in a single step has a concerted mechanism.
Regioselectivity arises from the charge distribution in the transition state. If boron becomes bound to the terminal the carbon bearing the R group is somewhat electron deficient (partial carbocation).
In contrast if the boron becomes bound to the carbon bearing the R group, then the terminal carbon is somewhat electron deficient in the transition state.
Because alkyl substitution at electron deficient carbon has stabilizing effect, the first transition state has the lower energy than the second. This has therefore a larger rate. Consequently, the first reaction is faster than the second and hence hydroboration is regioselective.
Oxidation to alcohols
Organoboranes can be converted into alcohols with hydrogen peroxide and aqueous sodium hydroxide.
The net result of this transformation is replacement of the boron by a OH in each alkyl group. The OH comes from the hydrogen peroxide. The net result, therefore, is the addition of elements of water to the double bond in a regioselective manner so that OH is bonded to the less branched carbon atom of the double bond.
Hydroboration-oxidation is the effective way to synthesize certain alcohols from alkenes. Especially of the general structure with a terminal OH group.
Because carbocaations are not involved in either the hydroboration of the oxidation reaction, the alcohol products are not contaminated by constitutional isomers from rearrangements.
Comparison of methods of alcohol synthesis
Hydration of alkenes is useful industrially. This is a specific method designed for special products and not general.
Hydroboration-oxidation and oxymercuration-reducton are both general laboratory methods for the preparation of alcohols from alkenes. A choice between the two methods usually hinges on the differences in their regioselectivities.
Hydroboration-oxydation gives an alcohol in which the OH group has been added to the less branched carbon of the double bond. Oxymercuration-redcution gives alcohols in which the OH group has been added to the more branched carbon of the double bond.
7. Ozonolysis
Ozone O3 adds to an alkene at low temperature yield an unstable compound called a molozonide. The molozonide is rapidly transformed into a second adduct called n ozonide. Note that both the C-C bonds of the double bond are broken in the formation of the ozonide.
The reaction of an alkene with ozone to yield products of double cleavage is called ozonolysis.
The first step is formation of the molozonide in another addition reaction of the alkene pi bond. The central O of the O3 is positively charged electronegative atom and therefore strongly attracts electrons.

The molozonide cycloaddition product is unstable and spontaneously forms the ozonide.
Ozonides can be converted into aldehydes, ketones or carboxylic acidic, depending on the structure of the alkene starting material and reaction conditions. When the ozonide is treated with dimethyl sulfide (CH3)2S the ozonide is split.
Note the transformation resulting from ozonolysis of alkene followed by dimethyl sulfide treatment is replacement of C=C group by two C=O
If the two ends of the C=C are identical then two equivalents of the same product are formed. If they are different then a mixture of the different products is obtained.
If the carbon of the double bond in the starting alkene bears a hydrogen, then an aldehyde is formed. In contrast, if the carbon of the double bond bears no hydrogen, then a ketone is formed instead.
If the ozonide is simply treated with water, hydrogen peroxide is formed as a by-product. Under these conditions (or if hydrogen peroxide is added specifically) aldehydes are convereted into carboxylic acids, but ketones are unaffected.
Summary of Ozonolysis Results under Different Coditions
Uses of Alkenes
In the presence of free-radical initiators such as peroxides many alkenes react to form polymers. Polymers are very large molecules composed of repeating units – monomers.
e.g. ethylene can be used as a monomer and polymerized under free-radical conditions to yield polyethylene.
This is an example of additional polymer.. Because the polymerization of ethylene occurs by a free-radical mechanism, it is an example of free-radical polymerization. The reaction is initiated when a radical R, derived from peroxides or other initiators, adds to the double bond of ethylene to form a new radica.
REFERENCES
Crocker,Ernest C (1992).Application of the Octet Theory To Single-Ring Aromatic Compounds
Macmurry,John(2007).Organic Chemistry(7th edition).Brooks-Cole
Organic Chemistry. Marc Loudon, 3rd edition The Benjamin/Cummings Publishing Company,Inc., California, 1995.
Organic Chemistry. John MucMurry. 3rd edition. Brooks/Cole Publishing Company, 1992



















No comments: