Systematic Inorganic Chemistry
Systematic Inorganic Chemistry
Group
IVA: C, Si, Ge, Sn
and Pb
The elements in group IVA; carbon-C,
silicon-Si, germanium-Ge, tin-Sn and lead-Pb. All members of group IVA exhibit an oxidation
state of +4, but the +2 oxidation state increases in stability down the group.The Ge(II) and Sn(II) states are well established, and Pb(II)
is more stable than Pb(IV) state. The elements possess the outer-shell electron
configuration of ns2np2.The electronegativities of the elements are generally low Formation of 4+ ions by electron loss is not
observed for any of these elements; the ionization energies are too high
Group IVA: General Properties
Property
|
|||
Atomic
radius (Å)
|
1st Ionization energy (kJ/mol)
|
X-X
single bond (kJ/mol)
|
|
C
|
0.77
|
1086
|
346
|
Si
|
1.17
|
786
|
226
|
Ge
|
1.22
|
762
|
186
|
Sn
|
1.40
|
709
|
151
|
Pb
|
1.46
|
716
|
-
|
C – strictly
non-metallic Ge – metalloid
Si – Essentially
non-metallic Sn & Pb – metallic
Carbon differs from the other group 4A
elements in its pronounced ability to form multiple bonds both with itself and
with other non-metals, especially N, O, and S.the strength of a bond between two atoms of a
given element decreases as we go down the group.Carbon-carbon bonds are quite strong. As a
consequence, carbon has a striking ability to form compounds in which carbon
atoms are bonded to one another.This property permits the formation of
extended chains and rings of carbon atoms and accounts for the large number of
organic compounds that exist. CARBON: Natural isotopes: 12C = 98.89% 13C = 1.11%
Radioisotope 14C
C4+ does not exist in any normal
chemical process.
C4- may possibly exist in some
carbides of the most electropositive metals (eg beryllium
carbide, Be2C, and aluminium carbide, Al4C3) in which the
high charge density cations Be2+ and Al3+ can form stable
lattices with such highly charged anion.
C ® Is22s2 2p2 ® Is2 2s1 2px1 2py1 2pz1 (sp3 hybridization)
In general carbon forms covalent
compounds
Group IVA: Occurrence Carbon is the only one to occur in the
elemental state as DIAMOND and GRAPHITE.Silicon – 2nd most abundant element in the earth’s crust
forming about 27.7%. (second to oxygen).
Silicates are present in rocks.
Common Sand = impure form of silica.
Glass = mixture of silicates.
Germanium is a rare element. It occurs in
traces in coal, in rare mineral argyrodite, 4Ag2S.GeS2, in
germinate, Cu3(Ge.Fe)S4, and
as a mixture in zinc and tin ores.Tin occurs mainly as cassiterite
or tin stone,SnO2.Lead as galena, PbS
Group
IVA: General Properties
Coordination number:Maximum coordination number of carbon = 4.
Because it can never accommodate more than 8 electrons in its valence shell.Other members:
Can
expand their octet due to the accessibilty of d- orbitals. Thus,
they can have higher coordination numbers and
form complex ions e.g., SiF62-, PbCl42- etc
Catenation
- Carbon
Formation of chains or rings of carbon atoms,
not only with single but also with multiple bonds; C-C-C, -C=C-C, -CºC- Conditions for catenation:
1. An element must have a valence
of at least two.
2. Must form strong bonds to
itself.
3. A kinetic inertness of the catenated compounds towards other
molecules or ions
NOTE: Carbon has the highest tendency to
catenate.
Si & S ® Next to carbon in catenation but far inferior to it. Thermal stability of –C-C-C– chains is due to the intrinsic
strength of the C-C bond.C-C bond is stable towards oxidation due to comparable energy
between C-C and C-O bondsTherefore given the necessary activation
energy Si-Si bonds will be converted to
Si-O bonds ,S-S bonds will also be converted
to S-O bonds
C-C = 346 kJ/mol
Si-Si = 226 kJ/mol
S-S = 226 kJ/mol
|
C-O = 336 kJ/mol
Si-O = 368 kJ/mol
S-O = 330 kJ/mol
|
Some Group Trends
1. Catenation – Important feature of the Group.
Extensive
chains occur in Si & Ge hydrides, up to Si6H14 and
Ge9H20,
Silicon halides and in Ge2Cl6.For Sn
& Pb
catenation occurs only in organo-compounds.There
is a smooth decrease in the tendency to catenate – the order is C>> Si>Ge=Sn>>Pb
This may be ascribed partly due to the
diminishing strength of the M-M bonds (M = C, Si, Ge, Sn
& Pb)
2. Down the group there is a steady decrease
in the M-C and M-H bond energies.
3. Strength of covalent bonds (M-X) with
other atoms decrease from Si to Pb
NOTE:
Inspite of
high Si-Cl or
Si-F bond energies, compounds containing these bonds are highly reactive.
Example:Si-Cl bonds are much more reactive than Si-C bonds
because, though stronger, they are much polar [Sid+-Cld-] rendering the silicon
more susceptible to attack by a nucleophile such as OH-.
Allotropes
of Carbon Allotropy occurs because of different crystal
structures and shapes in the solid state and/or combination of different
numbers of atoms into molecules.Carbon has several allotropes including Diamond, Graphite and
Fullerenes
Diamond and Graphite
Diamond and Graphite are
allotropes of Carbon ie. Different forms of the same element
They differ in their physical and
chemical properties due to difference in the arrangement and bonding of the
atoms.
Diamond:One of the hardest substances
known:Denser than graphite [D = 3.51
gcm-3; G = 2.22gcm-3]At 300 K and 1 Atm Graphite is the more stable allotrope.
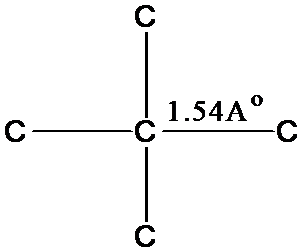
Diamond structureEach carbon atom is tetrahedrally
surrounded by four other carbon atoms at a distance of 1.54Å
covalently bonded to it.
Each C
atom is sp3 hybridized.The structure possesses no free electrons.Behaves as an insulator and is inert.The rigid, strong three dimensional linkages
make diamond one of the hardest substances known.
Graphite:
The structure of graphite is quite different
from that of diamond Amorphous carbons such as charcoal and soot
are very tiny particles of graphite ,Graphite consists of layers of carbon atoms Within the layers, covalent bonds
hold the carbon atoms in six-membered rings.The distance between the carbon
layers is very large (335 pm or 3.35 Å) Hence the attraction between layers is very weak .The layered structure of graphite
accounts for its ability to conduct electricity,,Observed softness and lubricity
is attributed to the easy slippage of these layers over one another
Note:
common structure of graphite = ABAB stacking
= Hexagonal form = MOST STABLE Every second layer is superimposable
Rhombohedral form –
stacking order = ABCABC
ie. Every third layer is superimposable.
Successful conversion of GRAPHITE ® DIAMOND
at
3000 K and pressures above 125 kbar.To obtain useful rates of
conversion, a Transition metal
catalyst [Cr, Fe or Pt] is used.
NOTE;40% of world’s supply of
Industrial quality diamonds are synthetic ones.Chemical reactivity of Diamond is
much lower than that of graphite due to the separation of the carbon sheets in
graphite
Some reactions of Graphite.
1. Mellitic acid (C6(CO2H)6: Obtained by treating graphite with hot
concentrated HNO3
2. Graphite
Oxide: Graphite reacts with a suspension of KClO4 in a 1:2 mixture (by volume) of conc. HNO3/H2SO4 to give graphite oxide.
This is an unstable pale lemon coloured
product of variable stoichiometry and structure. It decomposes slowly at 70 oC
3. Graphite monofluoride (CFx): Graphite reacts with an atmosphere of
fluorine at 400-500oC to give graphite monofluoride
CFx (x =
appr.
0.68-0.99).
The
reaction is catalysed by
HF and can then occur at much lower temperatures.
At ca.
600oC the reaction proceeds with explosive
violence to give a mixture of CF4, C2F6, and
C5F12.
The colour of CFx
depends on the reaction temp. and on the fluorine content (ie.
Black (x ≈ 0.7), Grey ( x ≈ 0.8), silver (x ≈ 0.9) and transparent white (x
> 0.98)). The structure has not been definitely established.
4. INTERCALATION COMPOUNDS OF GRAPHITE
Intercalation is the reversible inclusion of a molecule
(or group) between two other molecules (or groups). Example, graphite
intercalation compounds.
Graphite may undergo reactions in which the
carbon layers move further apart and molecules or ions are accumulated between
them; the products of such reactions are known as intercalation or lamellar compounds.The
reactions are often reversible and the graphite nature of the host lattice is
retained.Graphite,
which has good electrical conductivity ®the
conductivity remains and is sometimes enhanced
How
are intercalation compounds of graphite formed
Formed
by insertion of various atoms, molecules or ions between the layers of
graphite. Eg. K,
Na, F-, Br-,
FeCl3, BrF3, TiF4, etc.eg. C8K is formed by direct interaction of graphite
with K vapour at
300oC. Rb and Cs behave similarly. In
this compound, the graphite layers remain intact.
Note: The electrical resistance of graphite
intercalation compounds is lower than for graphite itself. In
contrast to graphite, which is diamagnetic, the compounds have a
temperature-independent paramagnetism.The graphite intercalation compounds formed
by the halides of different elements (ie. HF, ClF3, BrF3, XeF6, TiF4, FeCl3,
etc) are prepared by heating a mixture of the reactants.
INORGANIC
COMPOUNDS OF CARBON
1. CARBIDES:These are solid binary compounds in which
carbon combines with elements of similar or lower electronegativity, especially metals.
Obtained by direct interaction (or with metal
oxides) at high temp.
2Al2O3 + 6C ® Al4C3
+ 3CO2
Carbides of the most electropositive elements
such as the alkali and alkaline earth metals which mostly contain dicarbide ion
C22- (called acetylide) exhibit ionic bonding. They react with
water to produce ethyne, C2H2;
Na2 C2 + 2
H2O
→ 2NaOH + C2H2
CaC2 + 2H2O →
Ca(OH)2 + C2H2
However, the beryllium carbide (Be2C) and aluminium carbide (Al4C3) which contain C4- ion react with water to produce methane, CH4;
Al4C3 +
12H2O → 4Al(OH)3 +
3CH4
Whereas silicon and boron carbides (SiC
& B4C) exhibit covalent bonding. This is due to
the electronegativity differences, whereas most of nonmetals are more
electronegative than carbon.
2. OXIDES:
Four binary oxygen compounds of carbon are
known; CO, CO2, C3O2, C12O9:
i.
Carbon monoxide (CO) is formed when elemental carbon burns in a deficiency of
oxygen.
ii. Carbon dioxide (CO2) is formed when C burns in an excess of
oxygen.
iii. Carbon suboxide (C3O2) formed by dehydration of malonic acid
H2C(CO2H)2.
iv. Dehydration of benzenehexacarboxylic acid (mellitic acid) C6(CO2H)6
gives a carbon oxide (C12O9).
3. CARBON HALIDES:
All contain the carbon atom tetrahedrally coordinatined to the four
halogen atoms
CF4 – Extraordinary stable compound.
It
is the end product in the fluorination of any carbon containing compound.
CCl4
= Common solvent.
CBr4 and CI4 are also known.
There is steady decrease in the C–X bond
energies i.e., C-F>C-Cl>C-Br>C-I. This is because of size
factor.
4. Carbon also forms compds with C-N bond, [eg. HCN, (CN)2] and C-S bond,
[eg. CS2).
1. Hydrides, MH4 : M = Si ®Pb are covalent.
The stability of the hydrides decreases down
the group because of the decreasing M–H bond energies.PbH4 has
not been properly characterised .
SILANES : compounds with
Si-H bonds.
Silanes, SinH2n+2 are
chains of up to n = 8 and as cyclic compounds SinH2n (n = 5, 6)
Thermal stability decreases with increasing
chain length and only SiH4 (monosilane) and
Si2H6 are
indefinitely stable at 25oC. Higher silanes
decompose. Above 500⁰C all silanes decompose to Si and H2
The silanes react vigorously with oxygen and
spontaneously ignite or explode in air.
Silanes do
not react with pure water. However, In the presence of base, Si-H bonds are
rapidly hydrolyzed;
SiH4 + (n +1)H2O →
SiO2.nH2O +
2H2
GERMANES:
Germanes are
less flammable than silanes, although still rapidly oxidized in air.
Resistant to hydrolysis:
GeH4 is
unaffected by 30% NaOH.
Stannanes are
also known [SnH4 (stannane) is
the lowest member].
2. Halides MX4: M = Si ®Pb.
X = F,
Cl. Are the most important.
SiF4 only partially hydrolyzed by water
2SiF4 + 4H2O ® SiO2 + 2(H3O)+ +
[SiF6]2- +
2HF
Whereas SiCl4 is
rapidly hydrolyzed by water;
SiCl4 + 2H2O ® SiO2 +
4HCl
3. Oxygen compounds.
SiO2, GeO2, SnO2 and PbO2 are
known.
SiO2 = purely acidic, GeO2 = less acidic
SnO2 = amphoteric, PbO2 =
somewhat more basic.
ie Basicity
increases from SiO2 ® PbO2.
SiO2 – Unreactive towards Cl2, H2, acids and most metals at 25oC, but is attacked by F2, aqueous HF, alkali hydroxides and fused
carbonates
Silicon dioxide (SiO2)
Silicon dioxide, SiO2, is commonly known as silica.
Forms a giant or macromolecular (three
dimensional) structure in which each
silicon atom is surrounded tetrahedrally by four covalently bonded oxygen atoms.
Each oxygen is bonded to two silicon atoms.
However, the overall stoichiometry
or the silicon – oxygen ratio remains the same, i.e. SiO2.
Note; silicon dioxide and carbon dioxide share
the same type of formula, yet their properties are very different;CO2 is a
colourless gas at RT whereas SiO2 is a
solid which melts at 1600⁰C.C-O bond is much weaker than Si-OTheir differences can be explained by their
chemical structures; CO2 consists of individual molecules with double bonds
between the oxygen atoms and the carbon, O=C=O so as to satisfy the valences of
both C and O atoms WhereasSiO2 is a giant covalent structure
consisting of an extended network of Si and O atoms, single bonded to achieve
their valences
4. Some anionic species: MF62-, M = Si, Ge, Sn, Pb.
ie. SiF62-, GeF62-, SnF62- are known.
Silicon only forms fluoro
anions, normally SiF62-. It is very stable and accounts for the
incomplete hydrolysis of SF4 in water.
2SiF4 + 2H2O ® SiO2 +
SiF62- +2H+ +
2HF
THE DIVALENT STATE:
Si – divalent Si ® Thermodynamically unstable. SiF2, SiO, SiS, SiH2
& SiCl2 have been identified in high temperature
reactions.Ge
forms stable dihalides; GeF2, GeCl2 and
GeBr2.
Disproportionate
on heating.
2GeX2 ® GeX4 + Ge
Pb –
only Pb has
a well defined cationic chemistry.
REFERENCES
Bailar
, J. C. , Emeleus , H. J. , Nyholm , R. , and Trotman-Dickinson , A. F. ( 1973
). Comprehensive Inorganic Chemistry ,
Vol.
1 . Pergamon Press , Oxford . The chemistry of metals is extensively covered in
this fi ve-volume set .
Burdett
, J. K. ( 1995 ). Chemical Bonding in Solids . Oxford University Press , New York . An advanced book that
treats
many
of the aspects of structure and bonding in solids .
Cotton,
F. A., Wilkinson, G., Murillo, C. A., and Bochmann, M. (1999). Advanced Inorganic Chemistry , 6th ed. This
book
is the yardstick by which other books that cover the chemistry of the elements
are measured. Several
chapters
present detailed coverage of the chemistry of metals.
Everest
, D. A. ( 1964 ). The Chemistry of Beryllium . Elsevier , Amsterdam . A general survey of the chemistry,
properties,
and
uses of beryllium .
Flinn
, R. A. , and Trojan , P. K. ( 1981 ). Engineering
Materials and Their Applications , 2nd
ed. Houghton Miffl in ,
Boston
. Chapters 2, 5, and 6. This book presents an excellent discussion of the
structures of metals and the
properties
of alloys .
Greenwood
, N. N. , and Earnshaw , A. ( 1997 ). Chemistry
of the Elements . Butterworth-Heinemann , Oxford . Chapters
20–29.
This book may well contain more descriptive chemistry than any other single
volume, and it contains
extensive
coverage of transition metal chemistry .
Jolly
, W. L. ( 1972 ). Metal-Ammonia Solutions . Dowden, Hutchinson & Ross , Stroudsburg, PA . A collection
of
research
papers that serves as a valuable resource on all phases of the physical and
chemical characteristics of
these systems that
involve solutions of group IA and IIA metals .



No comments: