Electrophilic Aromatic Substitution
Electrophilic Aromatic Substitution
Based on its structure and properties, what kinds of reactions should benzene undergo? Are any
of its bonds particularly weak? Does it have electron-rich or electron-defi cient atoms?
Benzene has six o electrons delocalized in six p orbitals that overlap above and belowthe plane of the ring. These loosely held o electrons make the benzene ring electron rich, and so it reacts with electrophiles. Because benzene’s six o electrons satisfy Hückel’s rule, benzene is especially stable.Reactions that keep the aromatic ring intact are therefore favored.As a result, the characteristic reaction of benzene is electrophilic aromatic substitution—a hydrogen atom is replaced by an electrophile.
Reactivity Considerations of Aromatic Compounds
The benzene ring consists of a ring with p
electrons above and below
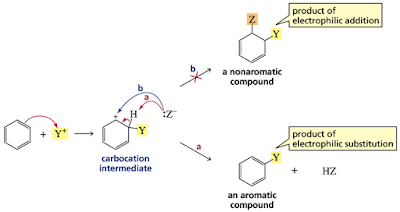
Electrophiles are attracted to a benzene ring
and form a nonaromatic carbocation intermediate (a cyclohexadienyl cation)
NOTE: Electrophilic addition doesn’t occur (would destroy aromaticity).Benzene does not undergo addition reactions like other unsaturated hydrocarbons, because addition would yield a product that is not aromatic. Substitution of a hydrogen, on the other hand, keeps the aromatic ring intact.
Mechanism for Electrophilic Substitution Reactions
The General Mechanism
No matter what electrophile is used, all electrophilic aromatic substitution reactions occur via a
two-step mechanism: addition of the electrophile E+ to form a resonance-stabilized carbocation,
followed by deprotonation with base, as shown in Mechanism blew•
Halogenation
The general mechanism outlined in Mechanism can now be applied to each of the fi ve specifi
c examples of electrophilic aromatic substitution shown in Figure . For each mechanism
we must learn how to generate a specifi c electrophile. This step is different with each electro-phile. Then, the electrophile reacts with benzene by the two-step process of Mechanism.
These two steps are the same for all fi ve reactions.
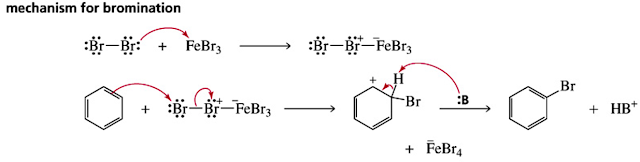
In halogenation, benzene reacts with Cl2 or Br2 in the presence of a Lewis acid catalyst, such as
FeCl3 or FeBr3, to give the aryl halides chlorobenzene or bromobenzene, respectively. Analogous
reactions with I2 and F2 are not synthetically useful because I2 is too unreactive and F2
reacts too violently.
In bromination (Mechanism ), the Lewis acid FeBr3 reacts with Br2 to form a Lewis acid–
base complex that weakens and polarizes the Br – Br bond, making it more electrophilic. This
reaction is Step [1] of the mechanism for the bromination of benzene. The remaining two steps
follow directly from the general mechanism for electrophilic aromatic substitution: addition of
the electrophile (Br+ in this case) forms a resonance-stabilized carbocation, and loss of a proton
regenerates the aromatic ring.
Always draw in the H atom on the carbon bonded to E. This serves as a reminder that it
is the only sp3 hybridized carbon in the carbocation intermediate.
• Notice that the positive charge in a given resonance structure is always located ortho
or para to the new C – E bond. In the hybrid, therefore, the charge is delocalized over
three atoms of the ring
Nitration
of Benzene
We must fi rst generate the electrophile and then write the two-step mechanism for electrophilic
aromatic substitution using it.
Any species with a lone pair of electrons can be used to remove the proton in the last step. In this
case, the mechanism is drawn with HSO4–, formed when +NO2 is generated as the electrophile.
Sulfonation
of Benzene
Nitration and sulfonation of benzene introduce two different functional groups on an aromatic
ring. Nitration is an especially useful reaction because a nitro group can then be reduced to an
NH2 group, a common benzene substituent,
Generation of the electrophile in both nitration and sulfonation requires strong acid. In nitration,
the electrophile is +NO2 (the nitronium ion), formed by protonation of HNO3 followed by loss
of water
Friedel–Crafts Alkylation and Friedel–Crafts Acylation
Friedel–Crafts alkylation and Friedel–Crafts acylation form new carbon–carbon bonds.
General Features
In Friedel–Crafts alkylation, treatment of benzene with an alkyl halide and a Lewis acid (AlCl3)
forms an alkyl benzene. This reaction is an alkylation because it results in transfer of an alkyl
group from one atom to another (from Cl to benzene
In Friedel–Crafts acylation, a benzene ring is treated with an acid chloride (RCOCl) and AlCl3
to form a ketone. Because the new group bonded to the benzene ring is called an acyl group, the
transfer of an acyl group from one atom to another is an acylation.
to form a ketone. Because the new group bonded to the benzene ring is called an acyl group, the
transfer of an acyl group from one atom to another is an acylation.•
Even with primary alkyl halides rearrangements occur via incipient primary carbocations.
The carbocation never really forms, but the
incipient carbocation remains complexed with the catalyst and behaves like a
primary cation.
Alkylation via Acylation Followed by Reduction
Problems associated with Friedel–Crafts
alkylation can be avoided by conducting an acylation followed by a reduction of
the carbonyl group to a methylene group (CH2).
The
method of reduction depends on other groups on the molecule
Two methods of reduction available;
1.0 Clemmensen reduction in acid solution
2.0 Wolff-Kishner reduction in basic solution
REFERENCES
Crocker,Ernest C (1992).Application of the Octet Theory To Single-Ring Aromatic Compounds
Macmurry,John(2007).Organic Chemistry(7th edition).Brooks-Cole
Organic Chemistry. Marc Loudon, 3rd edition The Benjamin/Cummings Publishing Company,Inc., California, 1995.
Organic
Chemistry. John MucMurry. 3rd edition. Brooks/Cole Publishing
Company, 1992
•


















No comments: